

Number 4, anything alkaline metals, anything in group 1, is going to be a +1 charge.
It is going to be a -2 charge, unless it's bonded to Fluorine, then it's going to be whatever it needs to be for Fluorine to be -1. Oxygen is the second most electronegative atom in the periodic table. Cl2, even though it has a 2 there, 2 has an oxidation number of 0, equal number of protons and electrons around that, in that particular atom.įluorine is extremely electronegative. So if you see aluminum by itself, it has an oxidation number of 0. So it's an overall charge of 0, including the diatomics.
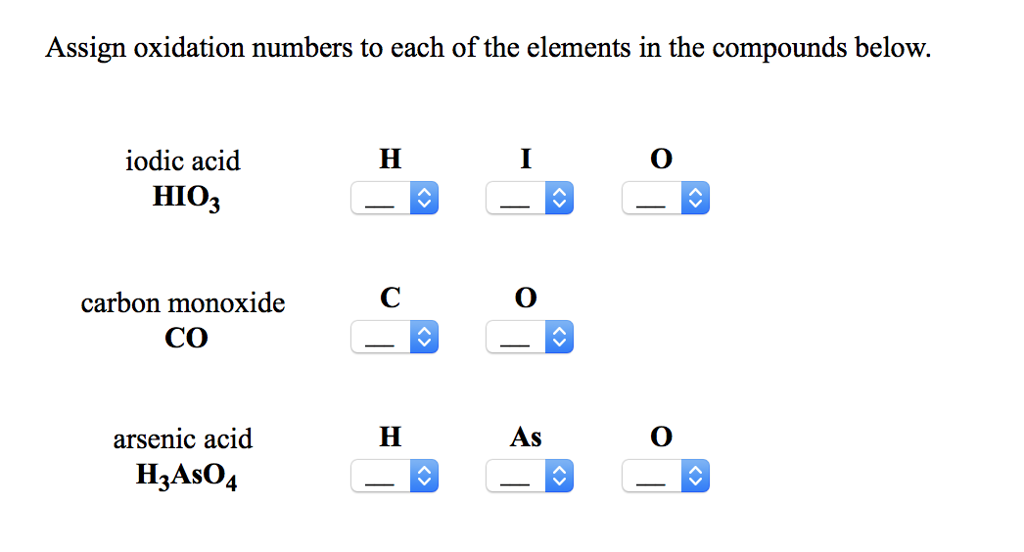
If an atom is by itself, looking at the first one, it has an awful time of spending, there's only one place electrons can spend and there's equal number of protons and electrons. So these are the rules we're going to follow, to make it easier for ourselves, to really understand what charge an atom is in a compound. They're very similar to charges when you're looking for actual charges on atoms and they're various too.
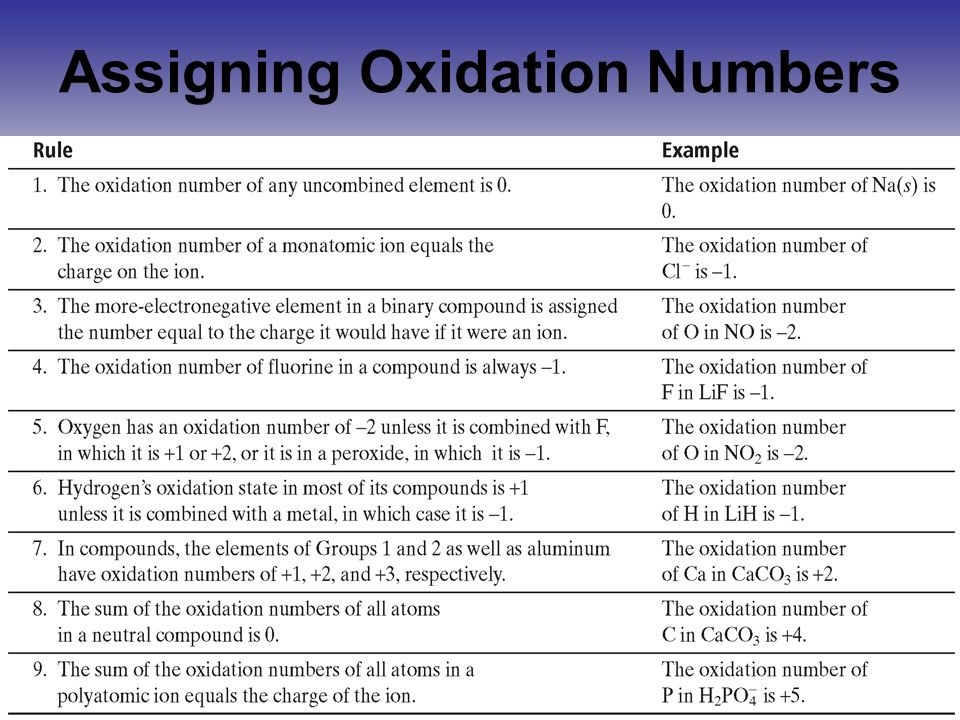
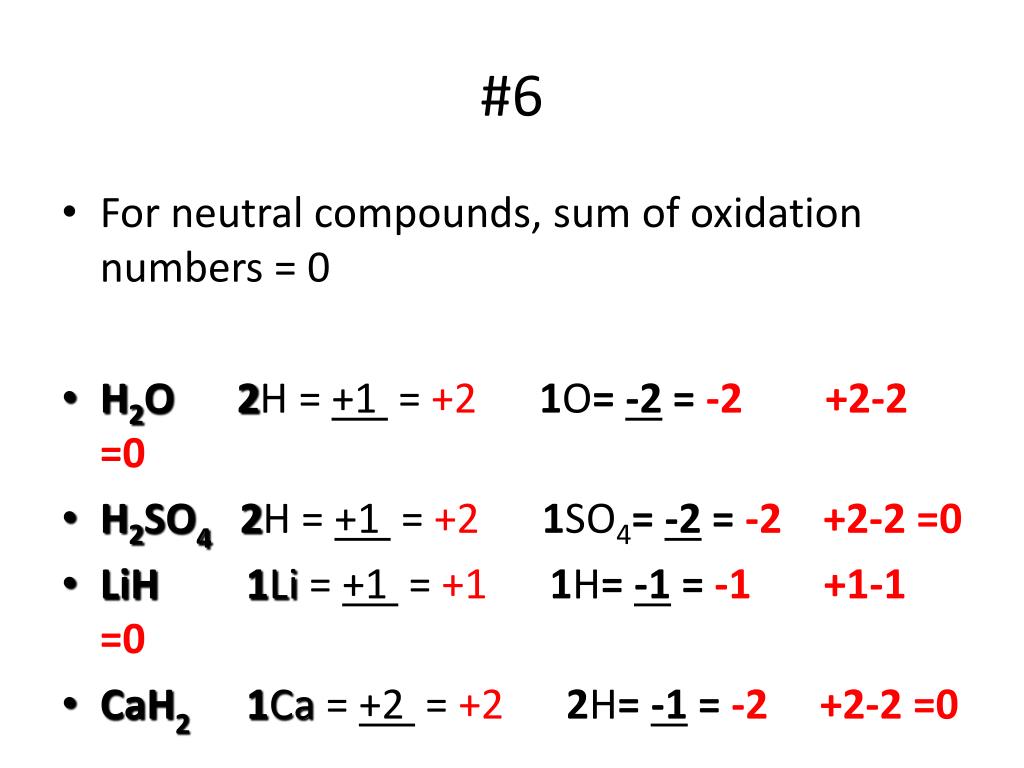
So these are relative numbers to decide where electrons are and where they're spending their time, basically. Well not most likely, definitely going to have the negative charge because electronegativity means those electrons are going to be around that particular atom more so, than the other atom in the compound. So the more electronic atom are most likely going to have a negative charge. Well, oxidation numbers are actually the relative charges that each of the atoms have in a chemical compound. What does it even mean? What are oxidation numbers? Oxygen in compounds usually have an oxidation number of $-2$, except in $\text$ ion so that all the charges on the ions add up to zero.So, when you're doing redox reactions, before you even get started, you're going to have to assign oxidation numbers to these compounds and atoms. Simple ions are given an oxidation number that is equal to its charge. The following table summarises the rules to assign oxidation numbers (O.N.): RulesĪll elements have an oxidation state of 0.


 0 kommentar(er)
0 kommentar(er)
